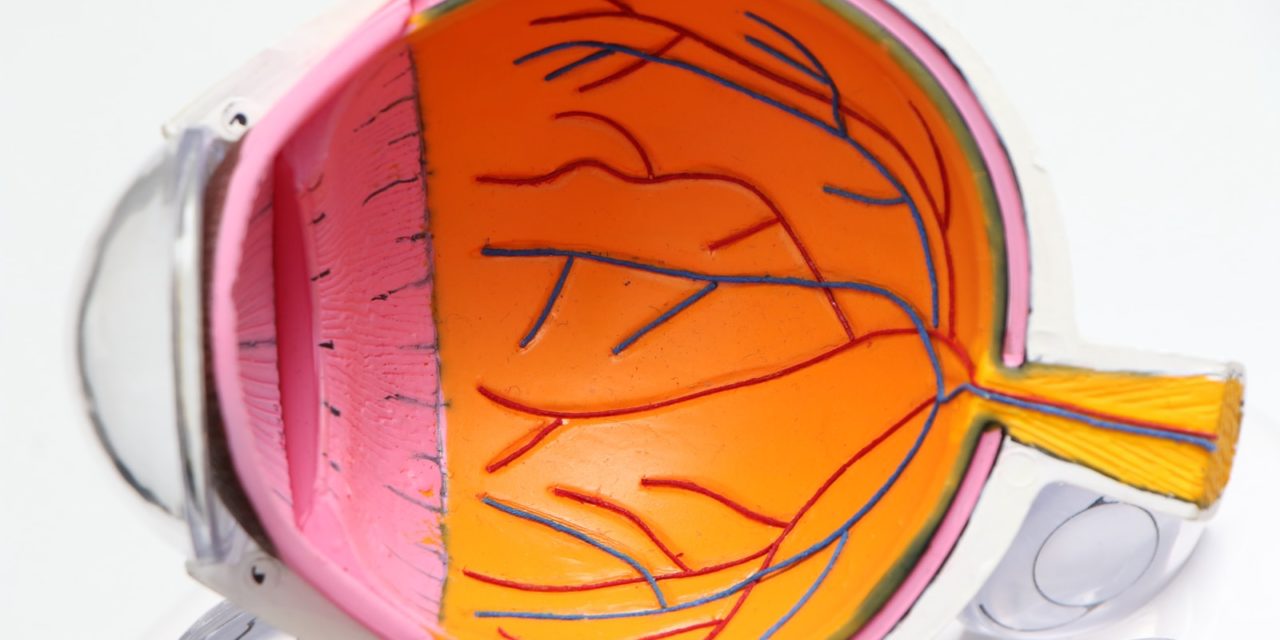
The Federal Food and Drug Administration approved the Federal Food and Drug Administration back in 2002. “no rub”lens solutions – One fluid that disinfects, cleans and removes protein deposits from contact lenses. Simply soak. FDA has been reexamining its policies after two cases of eye-disease that were linked to no-rub disinfectant.
In 2006, the Centers for Disease Control (CDC) linked one no-rub solution, Bausch & Lomb’s MoistureLoc, with 180 Fusarium fungal infections, which can and did cause blindness. Advanced Medical Optics was also recalled in 2007 after the CDC linked its no-rub disinfectant with a sight-threatening Acanthemoeba epidemic.
The FDA presented new proposals in June 2008 to address these outbreaks. These included a relabeling of packaging to suggest rubbing and tighter testing. The FDA may also begin testing contact solutions against a wider range of bacteria.
What is the reason no-rub solutions are so ineffective? Contact lens solutions can cause irritation so manufacturers only use low levels disinfectant. Many people who wear contact lenses, 34 million in total, don’t take the time to properly care for their eyes. This includes rubbing the solution into the lenses, cleaning them thoroughly before bed, changing the contacts every other day, following the prescribed wear limits, and disposing of disposable contacts as soon as possible.
Some new products can clean and disinfect contact lenses quickly. The LensComfort Ultrasonic Cleaning, Disinfecting & Storage Unit, for example, uses ultrasound technology to completely clean contact lenses in 15 minutes.
Ultrasound waves can be used to enhance the effectiveness of disinfectant solutions in studies. Contact wearers can use LensComfort’s FDA approved ultrasound device to combine antimicrobial technology and ensure their eye health. It is not necessary to wait for contact lenses to clean, disinfect, emulsify, or mix solutions.
For more information about the LensComfort Ultrasonic Cleaning, Disinfecting & Storage Unit, visit www.lenscomfort.net.